Sealed lead batteries
Sealed lead batteries
Within a Static Uninterruptible Power Supply – UPS, the only real perishable element is the battery it contains, which is most often overlooked.
This is why we have included this chapter with the aim of providing some basic information on the correct handling of batteries.
Electrical characteristics
Capacity
The capacity of a battery (Ah) is expressed as the product of the discharge current (A) and the time (h) elapsed until the final discharge voltage is reached.
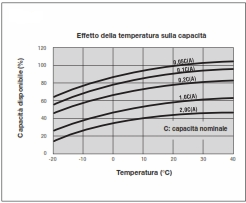
It varies in relation to the intensity of the current delivered.
The nominal capacity (C) is conventionally defined when discharged in 20 h, with a discharge end voltage of 1.75V / cell at a temperature of 20 / 25°C.
Discharge
To avoid reducing the life of a battery, it is recommended not to discharge it beyond the minimum voltages specified by the manufacturer.
The maximum continuous discharge allowed depends on the type of terminal installed on the battery (faston or screw/bolt terminal).
Conventionally, the maximum discharge current is indicated as 6 times the battery capacity in amperes.
The discharge of a battery is an electro-chemical reaction between the electrodes (the plates) and diluted sulphuric acid.
At relatively high discharge currents, or at low temperatures, when the viscosity of the acid rises and, consequently, its diffusion in the plates can no longer follow the discharge, the capacity decreases, as shown here.
Self-discharge
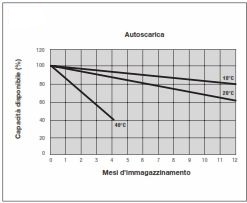
The loss of battery capacity over time is called self-discharge.
Thanks to the use of Pb-Ca alloys, this effect, due to sulphation of the plates, has been greatly reduced.
Therefore, batteries can be stored for a long time or used only occasionally.
Under normal conditions, at a temperature of about 20/25°C, the daily self-discharge is around 0.1% of the nominal capacity, about 25-30% less than conventional batteries.
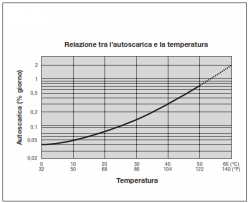
The relationship between self-discharge and temperature is shown above. The self-discharge doubles with every 10°C increase in temperature.
Open-circuit voltage
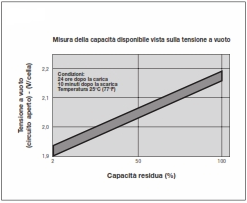
In conventional open batteries with liquid acid and topping up caps, the residual capacity can be estimated by measuring the density of the acid.
Since this is not possible with sealed batteries, the only way to determine the approximate residual capacity is by open-circuit voltage.
Approximately 24 hours after charging, or at least 10 minutes after discharging, the open-circuit voltage is measured and, with the help of the curve shown here, an indicative estimate of the residual capacity is obtained.
Battery life
When the battery has been used for a long time, the electrical capacity begins to deteriorate to the point where it can no longer be restored by charging.
This means that the battery has reached “its age limits”.
Since the life of the battery depends largely on the conditions of use, it is very difficult to predict how long it will last.
The main factors that negatively affect battery life are:
- Depth of discharge
- Amount of overcharge
- Charging current and voltage
During the charging phase, a high initial current can generate excessive heat. As a result, batteries, whether assembled or not, placed in an unventilated environment will deform (swell).
The same phenomenon can occur when the charging voltage is excessive. - Ambient temperature
The higher the ambient temperature, the greater the deterioration of the battery during operation.
Battery life in cyclic use
Initially, the capacity tends to increase as the plates are fully formed.
The number of cycles decreases as the depth of discharge increases.
Higher capacity batteries can be used for a longer time than lower capacity batteries when applied to the same load.

Battery life in buffer use
The width of the curve indicates the normal tolerance of the battery capacity. Since the life depends to a large extent on the charging voltage, it is necessary to remain within the limits of 2.25 – 2.30 V/el. (+ temperature compensation factor).
As can be seen from the figure, an increase in ambient temperature causes a significant reduction in service life.

Battery life in over-discharge
The life of a battery is greatly reduced if it is discharged too deeply or if it is stored discharged.The graph represents the relationship between the number of over-discharges and the percentage of the nominal capacity obtainable after recharging.

Operating Instructions
Assembly and connection
- Never charge batteries in a hermetically sealed container.
- Secure the battery well and protect it from vibration and shock.
- If the battery is installed in a cabinet, secure it well at the lowest possible level.
- Do not install the battery near sources of heat or possible sources of sparks.
- Temperature differences between installed batteries are quite normal. Make sure that the difference between all installed batteries does not exceed 3°C.
- Do not place in contact with objects containing plasticisers, organic solvents or soft PVC, as these could damage the ABS battery case.
- Do not compress and/or bend the terminals or overheat them (do not solder!).
- It is not recommended to use the batteries upside down.
- Install the batteries in a cool and ventilated environment.
- Leave sufficient space between the batteries to allow proper ventilation (10 mm if possible).
- Always use all batteries at the same time.
- Avoid using the batteries in environments where, due to temperature variations, condensation on the batteries is possible.
- In the case of batteries in series, first connect the batteries to each other and only then connect the series to the load.
- During transport and/or storage, batteries lose some of their capacity due to self-discharge. They must therefore be well recharged before installation.
Storage
- Since self-discharge increases rapidly with temperature, we recommend storing the batteries at a temperature between -20°C and +40°C.Before storing the battery, separate it from any electrical circuits and preferably place it in a cool, dry place.
- During storage recharge the battery at least once every six months.
- The battery also ages during storage, so it is recommended to use it as soon as possible.
General comments
- Do not short the terminals.
- Use a clean cloth to clean the batteries.
- Never use petrol, oil, solvents or anything else, nor rags soaked in the above.
- Avoid sparks or flames in the vicinity of batteries.
- Do not attempt to open the battery. If electrolyte (diluted sulphuric acid) comes into contact with skin or clothing, wash off immediately with water. If it comes into contact with the eyes, wash thoroughly and consult a doctor.
- Do not throw the battery into a fire, it could cause an explosion.
- Never use batteries of different capacities, brands or lifetimes, as differences in characteristics can cause damage to the battery and possibly also to the appliances in which they are installed.
- At the end of its life, the battery should not be disposed of with ordinary waste but handed over to authorised waste disposal companies.